East Kent leads the way in robotic trials to improve patients’ mobility. Patients from Scotland, Suffolk, London, Surrey, Essex and Berkshire are travelling to Kent to take part in a ground-breaking clinical trial at the Kent and Canterbury Hospital.

The trial, led by East Kent Hospitals Neuro-rehabilitation Director Dr Mohamed Sakel and consultant clinical and research neuro-physiotherapist Karen Saunders, uses the latest robotic technology to help people with progressive conditions such as multiple sclerosis re-learn how to walk again.
Results so far have been positive, with one man planning to walk his daughter down the aisle at her wedding next month thanks to his progress in the trial.
Dr Sakel said it had the potential to improve the lives of hundreds of patients.
He said: “At times, we encounter patients who have been told that nothing can be done and now we have an opportunity to give them the option of something that could make a real difference to their lives.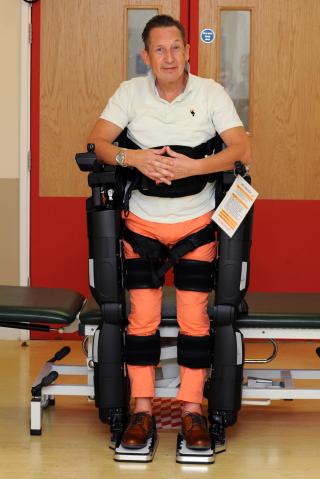
“We are enabling patients to rediscover muscles and movement that may have become somewhat lost over time. They are re-learning how to balance and to move more easily.
“The machine takes away the risk of falling and the fear that accompanies it and allows people to become confident in a safe environment. They can retrain their muscles and build up their strength so they are able to realise the benefits outside of the machine as well.”
During the trial, known as Rapper 4, patients are securely positioned into the machine, known as a Rex robotic exo-skeleton, and complete a programme of balance exercises.
This includes strengthening core abdominal muscles, lifting arm weights and throwing and catching a balloon. It also ‘walks’ them forward and back slowly, allowing them to focus on their core abdominal muscles to improve balance, mobility and strength.
The trial has recruited 20 patients and, when the results are analysed, it could become available to patients outside of research, if the benefits are proven.
Dr Sakel said: “We have to show it works, it is safe and maintainable and then we can recommend it.
“When we have research-based evidence we can begin to consider practice-based evidence. Obviously most people can’t have a machine at home – they cost in excess of £100,000 – but we could consider how to offer the benefits to hundreds of patients through our clinics.
“At the moment, a lot of the work around multiple sclerosis is focussed on patients who are already very disabled, but we know there are a lot of people living with the condition and simply adapting their lifestyles, such as moving to a bungalow or giving up work. Those are the people we believe we can help the most.”
Change and improvement is measured through a series of assessments before and after the five sessions on the device, including whether someone can stand without using their hands and how far they can reach without overbalancing.
The team is also gathering qualitative data such as patients’ opinions and experiences to help gain further insight and understanding of the effects and impact of the treatment. This could be used to potentially refine the design of the device for the future. The trial is due to finish in March 2020 and the data will then be analysed and reported.
Dr Sakel thanked the Kent MS Therapy Centre, Berkshire MS Therapy Centre, and the Chartered Society of Physiotherapy for their support.
The trial has had some great press coverage including: http://ow.ly/wRyx50vNCa7 and http://ow.ly/Q6Im50vNCa8
If you would like to take part please contact us and we will provide you with the contact details for those running the trial.
